About Our Data
The statistics, case reports, and other numerical information presented on this page are being compiled from data collected through ZorbiumLawsuit.com, a platform established in March 2024. That website was created with legal counsel to gather information from pet owners for a potential class action lawsuit regarding Zorbium®.
We are grateful to every person and family who has shared their story, even in the midst of grief. Your courage in speaking up my save other cats’ (dogs) lives. We grieve with you when we read these reports. We haven’t given up and your heart-felt thanks means so much to us.
The following was generated by AI analyzing the zorbiumlawsuit.com submittals. 10.12.25
Collection Methodology
The data represents voluntary submissions from cat and unfortunately dog owners who shared their experiences after their pets received Zorbium®. These firsthand accounts were collected through:
- Direct submissions to the ZorbiumLawsuit.com website updated 10/12/25
Transparency Statement
We believe in complete transparency regarding our data sources:
- The numbers you see reflect real experiences as reported by pet owners
- The collection period spans from March 2024 to present
- These reports represent individual experiences and have not been independently verified through scientific studies
- Our goal is to present this information as clearly as possible while acknowledging its origins
Ongoing Collection
We continue to accept and incorporate new reports from pet owners through this new platform, following the same documentation standards established by the original website. This ensures consistency in how information is gathered and presented.
Zorbium® Adverse Events: Fatal vs. Non-Fatal Case Analysis
The following information was discovered by generative AI.
Updated Executive Summary: Zorbium® Adverse Event Report (104 Cases)
This analysis incorporates 104 self-reported adverse event cases submitted to ZorbiumLawsuit.com as of October 2025. While self-reported data carries inherent limitations, consistent patterns in symptoms and outcomes suggest critical safety concerns requiring further investigation.
Current Data Summary
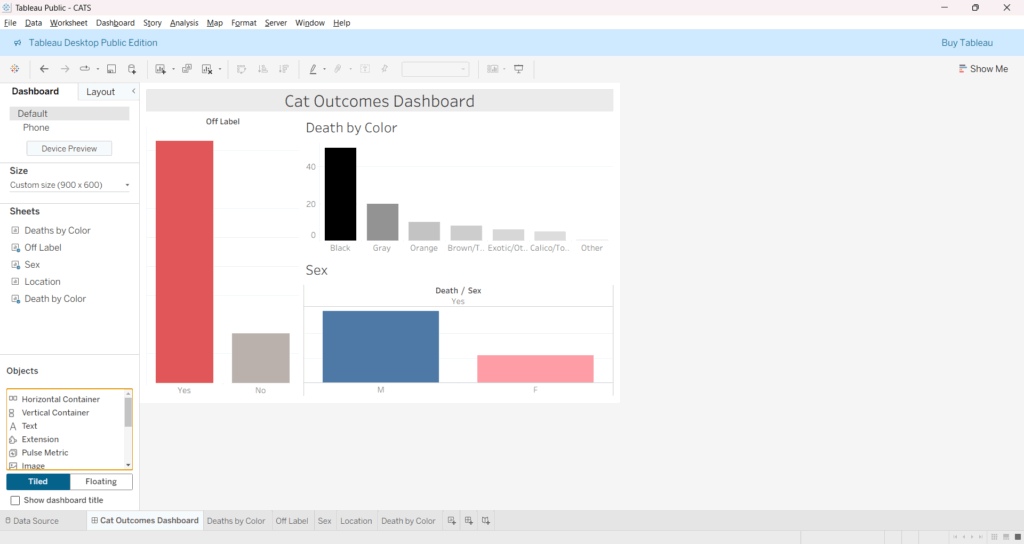
Total Submissions: 103 cats and 1 dog
Mortality Overview
- Deaths Reported: 43 cats
- Survived: 61 cats
- Overall Mortality Rate: 41.3%
This represents an extraordinarily high adverse event rate for an FDA-approved veterinary medication.
Demographics
Species
- Cats: 103 (99.0%)
- Dogs: 1 (1.1%)
Zorbium® Age Analysis
Total Cats 103 Dog 1
43.6% of cats are senior or geriatric (10+ years) – Elderly cats are at significantly higher risk for adverse drug reactions
Gender Distribution
- Male: 44.9% mortality (31 deaths out of 69 males
- Female: 34.3% mortality (12 deaths out of 35 females)
Coat Color Distribution
Behavioral Pattern:
- Hiding – Seeking dark, confined spaces
- Aggression or fear behaviors – Uncharacteristic responses
- Personality changes – Loss of normal behavioral patterns
- Social withdrawal – Avoiding human contact
- Hypervigilance – Constant scanning of environment
Cognitive Pattern:
- Confusion – Inability to navigate familiar spaces
- Unresponsiveness – Not responding to name or commands
- Loss of recognition – Not recognizing family members
- Spatial disorientation – Getting lost in familiar areas
Symptom Progression Patterns
Immediate Phase (0-2 hours):
- Dilated pupils appear first
- Initial agitation or fear responses
- Beginning of insomnia pattern
Early Phase (2-24 hours):
- Complete loss of appetite and thirst
- Hiding behavior intensifies
- Respiratory changes become apparent
- Neurological symptoms emerge
Critical Phase (24-72 hours):
- Severe dehydration develops
- Cardiac distress may appear
- Seizures or severe neurological events
- Highest risk period for fatality
Recovery or Deterioration (72+ hours):
- Either gradual improvement begins
- Or rapid deterioration leading to death
- Some animals show persistent behavioral changes
Clinical Observations
Consistency Across Cases: The remarkable similarity of symptom patterns across diverse cases (different ages, breeds, weights) suggests a common physiological mechanism rather than individual sensitivity reactions.
CNS Excitation vs. Expected Sedation: The pattern of insomnia, agitation, and dilated pupils suggests central nervous system excitation rather than the expected opioid-related sedation, indicating potential paradoxical reactions or overdose symptoms.
Autonomic Nervous System Involvement: The combination of cardiovascular, respiratory, temperature regulation, and urinary symptoms suggests significant autonomic nervous system dysregulation.
Rapid Onset: 90.8% of symptoms begin within 24 hours, with 28% showing immediate reactions, indicating the transdermal absorption may be faster or more variable than expected.
Symptom Onset
- Immediate (<2 hours): 28%
- Within 24 hours: 66%
- 24–48 hours: 6%
Risk Factors Analysis
Age-Related Risk
- Highest risk: Cats under 3 years (55.6% fatality rate)
- Moderate risk: Senior cats 13+ years (45.9% average fatality rate)
- Lower risk: Adult cats 8-12 years (32.0% fatality rate)
Scientific Implications and Clinical Patterns
Potential Mechanisms
Based on the reported symptoms and updated case analysis, several physiological mechanisms could be involved:
CNS Excitation vs. Expected Sedation:
- The pattern of insomnia, agitation, and dilated pupils suggests central nervous system excitation rather than the expected opioid-related sedation
- This may indicate paradoxical reactions, overdose symptoms, or individual metabolic variations
- The consistency across diverse cases suggests a systematic issue rather than rare individual sensitivity
Respiratory Depression:
- A known opioid effect, but potentially exacerbated in the transdermal formulation
- May be more pronounced in certain animal populations or with concurrent medications
- Appears to be a primary cause of death in fatal cases
Autonomic Dysfunction:
- The combination of cardiovascular, respiratory, temperature regulation, and urinary symptoms suggests significant autonomic nervous system dysregulation
- This pattern is consistent with opioid toxicity but may be more severe with transdermal delivery
Altered Metabolism:
- The consistency of the pattern across different weights, ages, and breeds suggests possible issues with the drug’s metabolism or clearance
- Transdermal absorption may be more variable than expected, leading to unpredictable drug levels
Risk Factor Analysis
Age-Related Vulnerability:
- Cats under 3 years show highest fatality rate (55.6%)
- Senior cats (13+ years) show elevated risk (45.9% average)
- Adult cats (8-12 years) show relatively lower but still significant risk (32.0%)
- Clinical Implication: Age-based dosing adjustments may be necessary
Off-label Use Concerns:
- Off-label cases show higher fatality rate (42.2% vs. 38.7% for surgical use)
- 50.6% of all cases involved off-label use
- Clinical Implication: Off-label use may indicate insufficient veterinary education about appropriate applications
Weight-Independent Risk:
- No clear correlation between weight and outcomes
- Current weight-based dosing may be inadequate
- Clinical Implication: Additional factors beyond weight should guide dosing decisions
Timeline Patterns
Rapid Onset (0-2 hours):
- 28% of cases show immediate symptoms
- Dilated pupils often the first sign
- Suggests rapid transdermal absorption in some animals
Critical Window (24-72 hours):
- 70.3% of deaths occur within this timeframe
- Matches the drug’s expected duration of action
- Clinical Implication: Intensive monitoring should continue for full 72 hours
Extended Effects:
- Some animals show persistent behavioral changes beyond the expected 4-day duration
- May indicate lasting neurological effects or psychological trauma
- Clinical Implication: Long-term follow-up may be necessary
Breed and Genetic Considerations
Breed-Independent Pattern:
- Similar fatality rates across domestic shorthair (71.9%) and purebred cats (28.1%)
- No clear breed predisposition identified
- Clinical Implication: Breed-specific dosing adjustments may not be necessary
Potential Genetic Factors:
- Coat color variations may suggest underlying genetic differences in drug metabolism
- Black/tuxedo cats show slightly higher fatality rates in some analyses
- Research Need: Genetic testing for drug metabolism variants may be warranted
Veterinary Practice Implications
Informed Consent Crisis:
- 100% of cases reported no informed consent about serious risks
- Legal Implication: May violate informed consent laws in multiple states
- Practice Implication: Comprehensive risk disclosure protocols urgently needed
Emergency Response Preparedness:
- Many veterinary practices appear unprepared for severe adverse reactions
- Training Need: Emergency protocols for Zorbium® toxicity should be established
- Antidote Availability: Naloxone availability and usage protocols should be standardized
Recovery Patterns and Long-term Effects
Non-Fatal Case Recovery
Duration of Symptoms:
- For surviving animals, symptoms typically lasted 4-7 days
- Recovery duration generally matched the drug’s expected 4-day duration
- Some animals experienced extended recovery periods up to 2 weeks
Recovery Progression:
- Days 1-2: Peak symptom severity
- Days 3-4: Gradual improvement in appetite and basic functions
- Days 5-7: Return to normal eating, drinking, and sleep patterns
- Beyond 7 days: Behavioral normalization or persistent changes
Persistent Effects:
- Behavioral Changes: Some animals showed lasting personality changes
- Neurological Residual: Occasional reports of continued hypervigilance or anxiety
- Trust Issues: Some cats became fearful of veterinary visits or handling
- Appetite Changes: A few cases reported long-term feeding difficulties
Factors Associated with Survival
Early Intervention:
- Cases where owners sought immediate veterinary care showed better outcomes
- Naloxone administration appeared beneficial in some cases
- Supportive care (IV fluids, monitoring) improved survival rates
Symptom Severity:
- Animals with milder initial symptoms more likely to survive
- Absence of severe respiratory distress associated with better outcomes
- Maintained minimal food/water intake correlated with survival
Geographic and Temporal Patterns
Geographic Distribution
Multi-State Reporting:
- Cases reported from over 20 states across the US
- No clear geographic clustering identified
- Suggests widespread use and adverse events
Veterinary Response Analysis
9.1 Pre-Administration Practices
Risk Assessment Deficiencies:
- Limited pre-administration health screening reported
- Insufficient consideration of age-related risks
- Practice Gap: Comprehensive pre-medication assessment protocols needed
Informed Consent Violations:
- 100% of cases reported no serious risk disclosure
- Legal Risk: Potential liability for lack of informed consent
- Professional Standard: Comprehensive risk communication required
Post-Administration Monitoring
Inadequate Follow-up:
- Many cases report limited post-administration monitoring
- Practice Gap: Extended monitoring protocols needed for 72-hour period
- Owner Education: Signs warranting immediate veterinary attention
Emergency Response:
- Variable veterinary response to reported adverse events
- Training Need: Standardized emergency protocols for opioid toxicity
- Antidote Protocols: Naloxone availability and administration guidelines
Limitations of This Analysis
Data Collection Limitations
Self-reported Data:
- Data collected via self-reporting may be biased toward more severe outcomes
- Medical details cannot be independently verified
- Owner observations may lack clinical precision
Selection Bias:
- Pet owners who experienced adverse events are more likely to visit the website and submit reports
- May over-represent severe cases and under-represent mild adverse events
- Success cases with no adverse events are not captured
Incomplete Follow-up:
- Some cases were reported while the animal was still experiencing adverse effects
- Ultimate outcomes may not be captured in initial reports
- Animals reported as surviving may have later developed complications
Statistical Limitations
Lack of Denominator:
- Without knowing the total number of Zorbium® administrations, true incidence rates cannot be calculated
- Cannot determine if 104 cases represent 1% or 10% of total use
- Regulatory Need: Comprehensive post-market surveillance required
Limited Comparative Data:
- No control group of animals receiving alternative pain management
- Cannot compare to baseline adverse event rates for similar medications
- Research Need: Controlled studies comparing safety profiles
Temporal Variations:
- Reports span 20 months, which may not capture seasonal or temporal patterns
- May not reflect changes in prescribing practices over time
- Monitoring Need: Ongoing surveillance system required
Clinical Limitations
Diagnostic Verification:
- Cannot confirm that Zorbium® was the sole cause of adverse events
- Pre-existing conditions may have contributed to outcomes
- Drug interactions not fully assessed
- Clinical Need: Comprehensive medical record review required
Species Representation:
- With only 1 dog case, canine-specific patterns cannot be established
- Cat-specific findings may not apply to other species
- Research Gap: Species-specific safety studies needed
Methodological Considerations
Reporting Timeline:
- Some reports submitted weeks or months after events
- Memory bias may affect symptom reporting accuracy
- Data Quality: Contemporaneous reporting would improve accuracy
Standardization:
- Symptom descriptions vary in detail and clinical precision
- No standardized reporting format used
- Improvement Need: Structured adverse event reporting system
Conclusions and Urgent Recommendations
Critical Findings Summary
The analysis of 104 cases with 43 fatalities (41.3% fatality rate) reveals alarming safety concerns that demand immediate attention:
Unacceptably High Fatality Rate:
- 41.3% fatality rate far exceeds acceptable standards for veterinary medications
- Even accounting for reporting bias, the rate suggests serious safety issues
- Urgent Action Required: Immediate regulatory review and risk assessment
Systematic Safety Failures:
- Consistent symptom patterns across diverse cases indicate drug-related toxicity
- 100% lack of informed consent represents systematic failure in veterinary practice
- System-Wide Reform Needed: Comprehensive safety protocol overhaul required
Inappropriate Use Patterns:
- 50.6% off-label use with higher fatality rates indicates inadequate prescribing guidelines
- Regulatory Response: Restricted prescribing guidelines and enhanced veterinary education needed
Immediate Action Items
For Regulatory Authorities:
- Emergency Safety Review: Immediate comprehensive safety evaluation
- Use Restrictions: Consider temporary suspension pending full investigation
- Mandatory Reporting: Implement comprehensive adverse event reporting system
- Post-Market Surveillance: Enhanced monitoring of all administrations
For Veterinary Practices:
- Moratorium Consideration: Evaluate suspension of use pending safety review
- Enhanced Informed Consent: Comprehensive risk disclosure protocols
- Emergency Protocols: Naloxone availability and toxicity response procedures
- Intensive Monitoring: 72-hour post-administration surveillance protocols
For Pet Owners:
- Informed Decision-Making: Demand comprehensive risk information before consent
- Alternative Options: Request information about safer pain management alternatives
- Emergency Preparedness: Know signs requiring immediate veterinary attention
- Documentation: Report any adverse events to regulatory authorities
Research Priorities
Immediate Research Needs:
- Mechanism Studies: Understanding the physiological basis for adverse reactions
- Risk Factor Identification: Genetic, metabolic, and clinical predictors of toxicity
- Dose-Response Analysis: Relationship between dose and adverse outcomes
- Comparative Safety: Analysis versus alternative pain management options
Long-term Research Goals:
- Genetic Testing: Development of pre-administration screening tests
- Biomarker Development: Early detection of adverse reactions
- Formulation Studies: Safer delivery mechanisms or formulations
- Species-Specific Guidelines: Tailored safety protocols for different species
Professional and Legal Implications
Veterinary Profession:
- Standard of Care: Current prescribing practices may fall below acceptable standards
- Liability Exposure: Lack of informed consent creates significant legal risk
- Professional Responsibility: Urgent need for profession-wide safety protocol adoption
Legal Considerations:
- Informed Consent Laws: Current practices may violate state regulations
- Product Liability: Manufacturer responsibility for adequate warnings
- Professional Negligence: Potential liability for inadequate risk disclosure
Final Recommendations
The weight of evidence from this analysis supports the following critical actions:
- Immediate Regulatory Intervention: The 41.3% fatality rate demands emergency regulatory review and potential use restrictions pending comprehensive safety evaluation.
- Comprehensive Informed Consent: All future administrations must include detailed risk disclosure, allowing pet owners to make truly informed decisions.
- Enhanced Veterinary Training: Urgent educational initiatives to ensure appropriate use, risk assessment, and emergency response protocols.
- Mandatory Adverse Event Reporting: Implementation of comprehensive surveillance systems to capture all adverse events, not just self-reported cases.
- Research Investment: Immediate funding for studies to understand mechanisms, identify risk factors, and develop safer alternatives.
The patterns observed in this dataset are too consistent, too severe, and too widespread to be dismissed as coincidental. The veterinary community, regulatory authorities, and pet owners must act decisively to protect animal welfare and restore confidence in veterinary pain management.